What we do
We combine quantitative experiments with computational methods to understand the emergence of multicellular organization.
Cells are able to organize themselves to form multicellular structures and to coordinate in collective processes. This is perhaps most evident during embryonic development, where the organized differentiation and movement of multiple cell lineages ends in the formation of viable individuals. However, cell groups are also able to self-organize in pathological and seemingly chaotic conditions such as during cancer progression. We want to understand the fundamental mechanisms behind multicellular organization during both physiological and pathological processes.
Our long-term goal is to describe, understand and eventually control cell organization and collective cellular processes. We think that this knowledge will help us understand more deeply the emergence of collective behavior in nature and could inspire novel approaches to look at deranged multicellular activities.
We think that a large part of this organization can be understood as the result of social and ecological interactions between cells. In these interactions, metabolites -nutrients and waste products of cellular activity- ought to play a critical role, so we are paying special attention to cell metabolism as well as the chemical composition of the cellular environment. To study this, we combine experimental and computational methods trying to answer two fundamental questions:
-
How do neighboring cells influence each other?
-
What are the reciprocal interactions between cells and their environments?
Ecology of tumor-stroma interactions: competition and cooperation among tumor cells
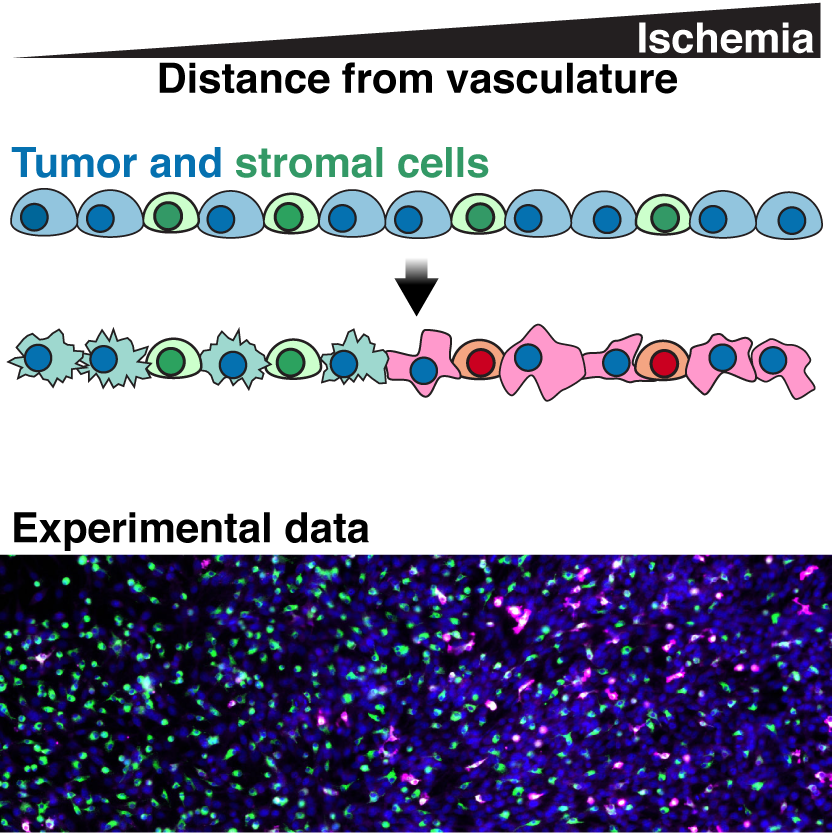
The microenvironment experienced by cancer cells plays a fundamental role in how malignant they become. Understanding how tumor cells integrate different external signals is necessary to predict and eventually control cancer progression. We want to explore how metabolic features of the tumor microenvironment, such as low pH and nutrient scarcity, interact with cell-to-cell communications to determine tumor growth.
Two features of the microenvironment liken it to an ecological system. First, extracellular conditions, such as nutrient availability and oxygen levels, are not homogeneous. The exacerbated growth and deranged metabolism of cancer cells alter the chemical composition of the extracellular space while poor blood perfusion is insufficient to properly re-establish physiological levels. This creates gradual changes of extracellular metabolites that lead to a spatially-structured environment. Ecologists have been dealing with this type of problems for a long time, as gradual changes in altitude, temperature or atmospheric pressure lead to structured environments for animals and plants. Second, just like species in the wild are not isolated but form a community with other species, in the tumor, cancer cells are not alone. Tumors are heavily infiltrated by immune cells, fibroblasts, endothelial and other stromal cells. These are constantly interacting with each other, and such interactions can also be understood from an ecological standpoint.
We are investigating how different cell types interact with each other. In particular we are focused on tumor-macrophage interactions, but we are also interested in looking at tumor interactions with other cell types such as lymphocytes. How these interactions change in time and space has been largely ignored, partially due to the lack of adequate experimental models to do so. To advance on this front, we are using a multidisciplinary approach including the development of a custom–made microphysiological system designed to study the effect of graded environments on cell phenotypes and interactions. Read more about this system here. You can also check our publications page.
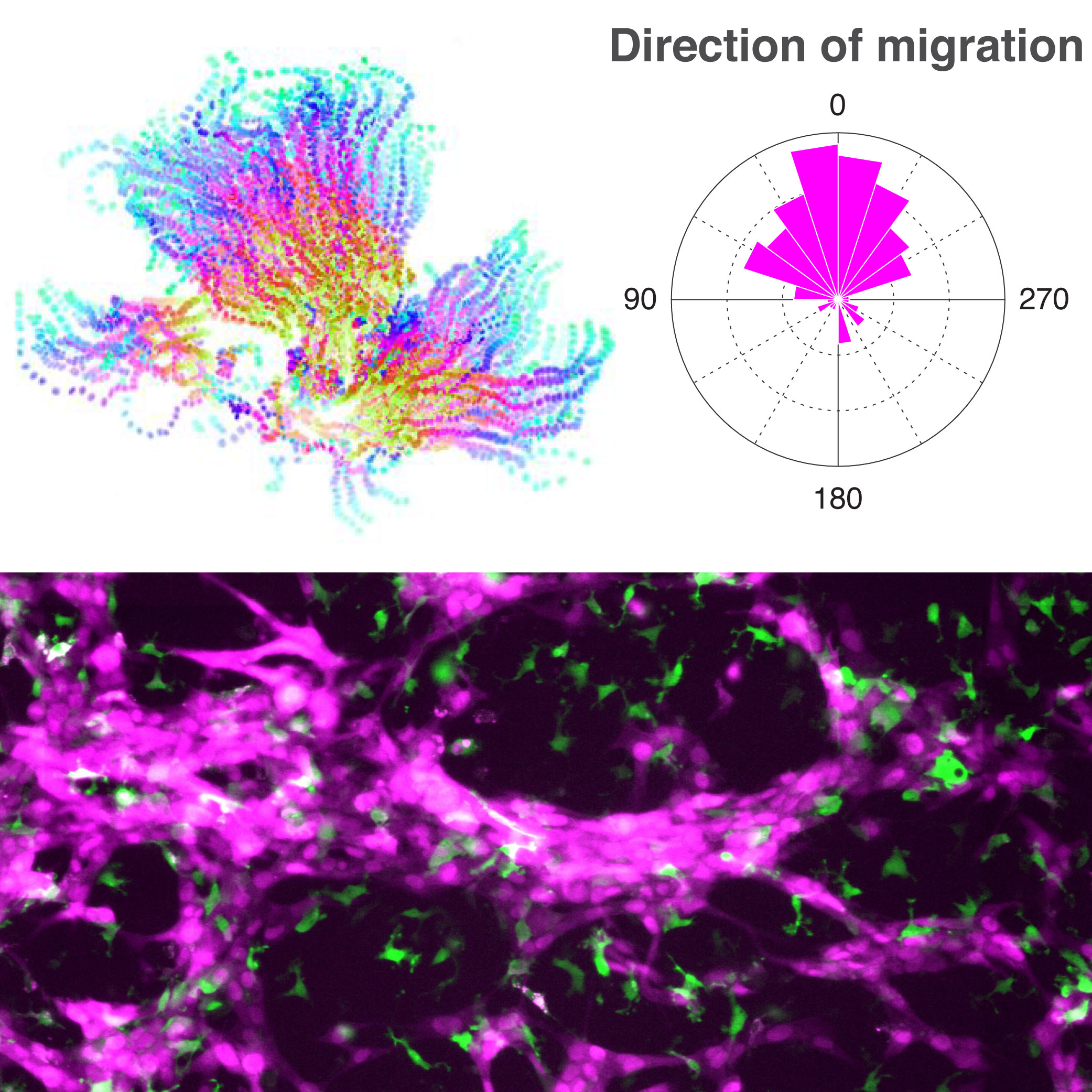
2. Decision making during collective migration in cancer and development
Cell migration is essential for animal morphogenesis and adult functions such as tissue repair and immune response. Unfortunately, cell migration is also a critical feature of tumors and indeed it is the dispersion of tumors, or metastasis, what put our lives at risk. We have learnt that often cells do not migrate alone but form groups that move in a cohesive manner. These cell groups have to take collective decisions such as where to go, how to invade a new tissue or if the group should form some kind of structure. How do they do this? We are investigating the role of nutrients in these and other collective decisions moving cells have to make. We hope to uncover fundamental mechanisms behind collective cell migration and potentially learn important lessons about tumor dispersion.
Read more about our work on cell migration in our publications page.
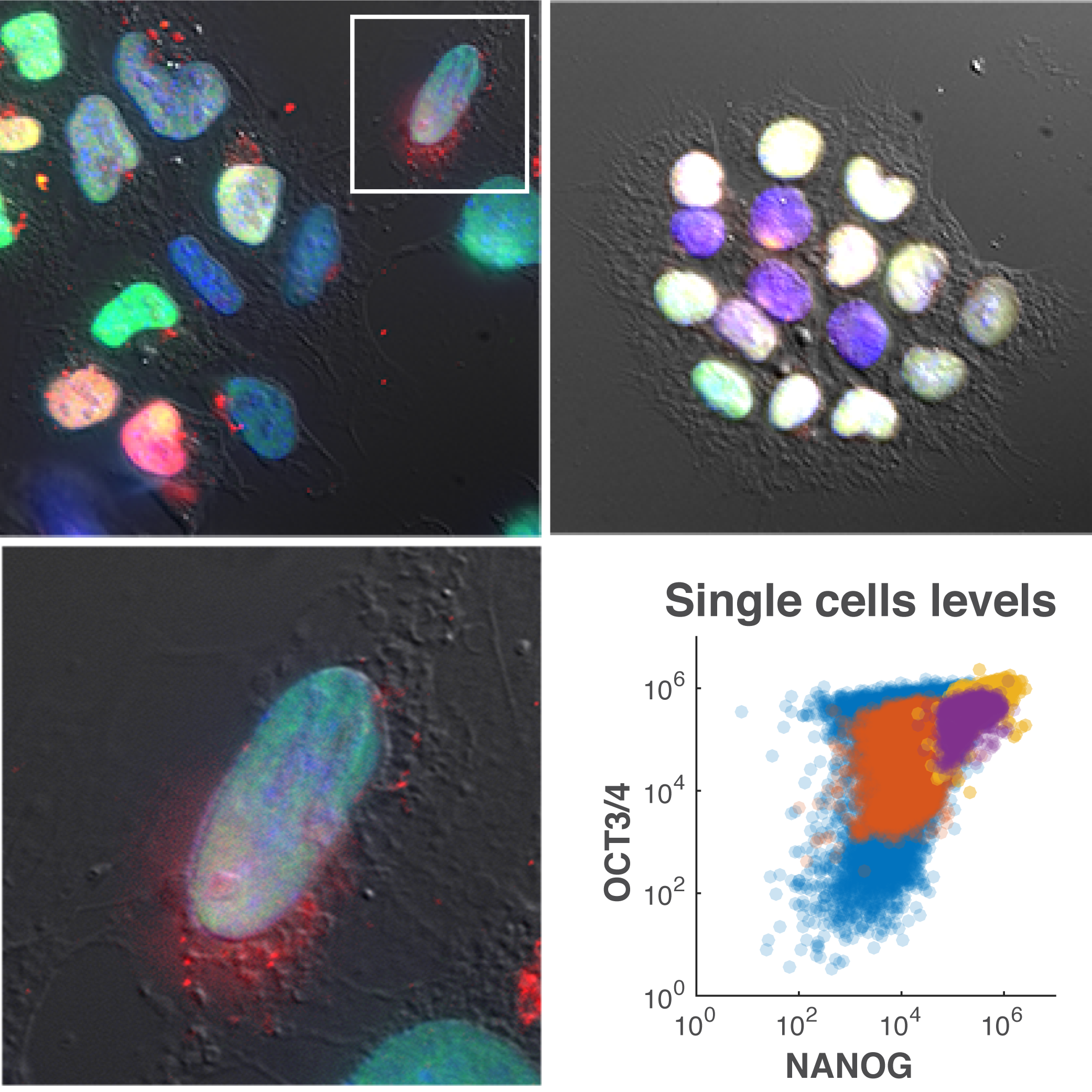
3. Metabolites in stem cell and embryonic organization
We are applying similar concepts that we use to understand tumor organization, to study stem cell maintenance and differentiation. Cell signals such as growth factors and cytokines are well known to modulate cell differentiation. However, little is known about the role of metabolites in this process.
We are starting by studying how metabolites control the multicellular organization and phenotypes of stem cells. Eventually, we would like to determine the influence of metabolites in embryonic differentiation. Although this research is still preliminary, we think that cell signaling pathways are heavily modulated by the concentrations of nutrients and thus their interplay is what determines the proper formation of embryos.
Read more about our research in development in the publications page.